Introduction
Nuclear Magnetic Resonance (NMR) spectroscopy is one of the most reliable and informative techniques used to study the internal structure of bioactive glass. Unlike crystalline materials, bioactive glasses are amorphous, meaning they do not have a regular, repeating atomic arrangement. Because of this lack of long-range order, traditional techniques such as X-ray diffraction are limited in the kind of structural information they can provide.
Bioactive glass was originally developed for biomedical uses such as bone repair and regeneration. Its ability to interact with living tissue comes from its atomic-level structure, which controls how the glass dissolves, releases ions, and bonds with bone. These processes are governed not by crystal structure, but by short-range atomic arrangements within the glass network.
This is where solid-state NMR becomes essential. NMR is particularly suited for studying disordered materials because it provides element-specific information and directly probes the local chemical environment around individual atoms. For bioactive glass research, NMR allows scientists to understand how atoms are connected, how modifier ions behave, and how these structural features influence biological performance.
Principle of NMR
When a bioactive glass sample is placed inside a strong magnetic field, certain atomic nuclei within the material respond to that field. These nuclei can align either with or against the magnetic field, creating different energy states. When radiofrequency energy is applied, the nuclei move between these energy states, producing an NMR signal.
The exact frequency at which this signal occurs depends on the electronic environment surrounding each nucleus. Electrons partially shield nuclei from the magnetic field, and this shielding changes depending on how the atom is bonded and which neighboring atoms are present. This shift in resonance frequency is known as the chemical shift.
In bioactive glass, chemical shifts provide direct insight into how the glass network is built. For example, NMR can reveal how silicate units are connected, how phosphate or borate groups are coordinated, and how modifier ions influence the structure. This makes NMR a powerful tool for linking glass composition to function.
Qⁿ Species and Bioactivity
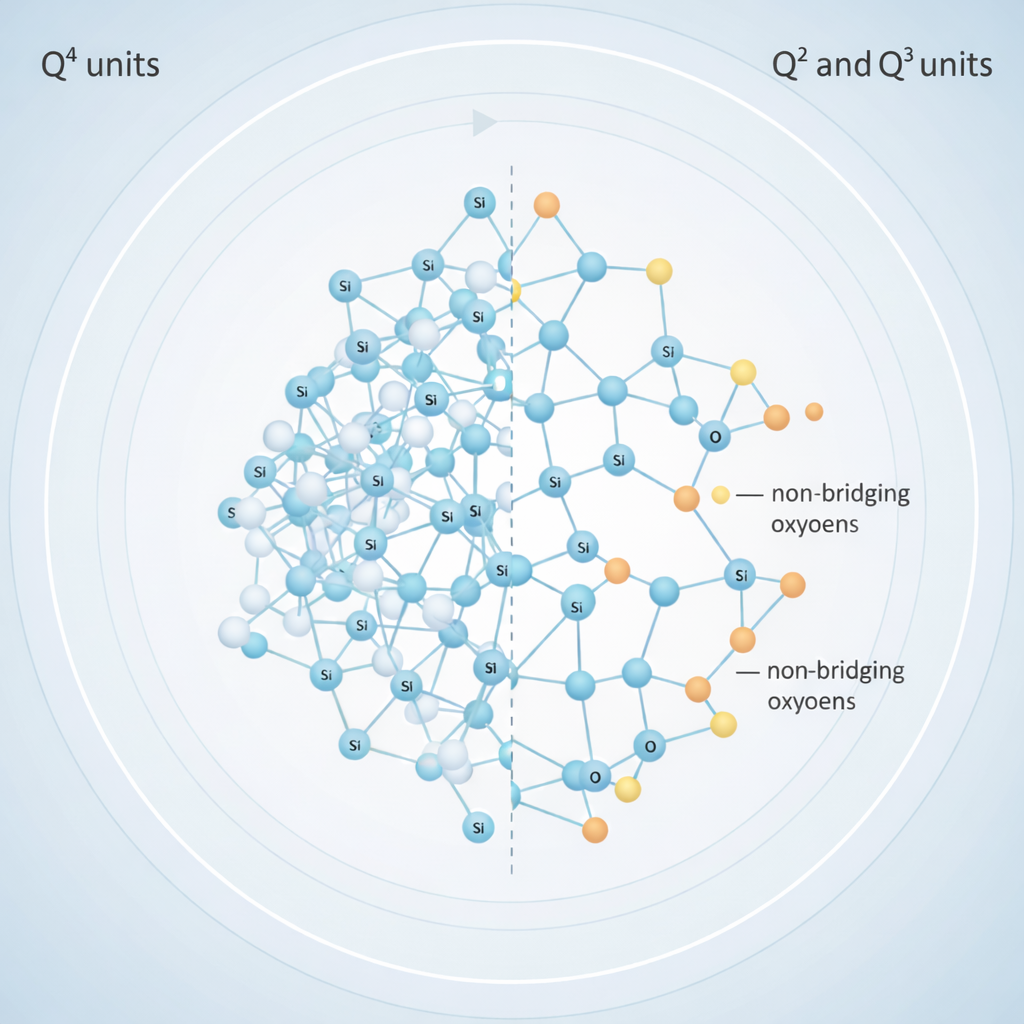
In silicate-based bioactive glass, one of the most important structural descriptions comes from ²⁹Si NMR, which classifies silicon environments using the Qⁿ notation. Here, “n” represents the number of bridging oxygens connected to a silicon atom within a SiO₄ tetrahedron. Any remaining oxygens are called non-bridging oxygens, and these are balanced by modifier ions such as calcium or sodium.
This Qⁿ classification gives a clear picture of how connected or disrupted the glass network is.
- Q⁴ units represent fully connected silicate structures with four bridging oxygens. These networks are highly rigid and chemically stable, meaning they dissolve very slowly in water. Glasses dominated by Q⁴ units tend to be bioinert, showing limited ion release and minimal interaction with body fluids.
- Q³, Q², and Q¹ units represent increasingly disrupted structures with more non-bridging oxygens. Bioactive glasses typically contain a higher proportion of Q² and Q³ species. This less-connected network allows water to penetrate the glass more easily, promoting ion exchange and controlled dissolution in physiological environments.
The balance between these Qⁿ species is therefore directly linked to bioactivity. A disrupted network encourages surface reactions and bone bonding, while an overly connected network reduces biological interaction.

Solid-State NMR for Amorphous Glasses and the Role of MAS
Solid-state NMR is especially valuable for studying bioactive glass because it works directly on solid materials without requiring crystallinity. This makes it suitable for analysing silicate, phosphate, borate, and complex multicomponent glasses used in biomedical applications.
A key technique used in solid-state NMR is Magic Angle Spinning (MAS). In MAS, the glass sample is rapidly spun at an angle of 54.74 degrees relative to the magnetic field. This specific angle averages out interactions that normally cause severe signal broadening in solids.
In amorphous materials like bioactive glass, atoms experience strong directional interactions that blur NMR signals. MAS reduces these effects, resulting in sharper and more readable spectra. This improvement in resolution allows researchers to clearly distinguish different structural environments within the glass.
Why Magic Angle Spinning Is Critical for Bioactive Glass Analysis
The use of MAS in solid-state NMR provides several major advantages when studying bioactive glass:
1. Clear Identification of Structural Units
MAS makes it possible to resolve individual Qⁿ species within silicate networks. Without MAS, these signals would overlap and become indistinguishable. By separating Q⁴, Q³, Q², and Q¹ environments, researchers can accurately assess network connectivity and predict dissolution behaviour.
2. Understanding Modifier Ion Behaviour
Modifier ions such as sodium, calcium, and magnesium play a critical role in bioactive glass performance. MAS improves the resolution of NMR signals from these ions, allowing their coordination and mobility to be studied. This information is essential for understanding ion release rates and surface reactions that drive bone bonding.
3. Analysis of Highly Disordered Systems
Because MAS reduces disorder-related broadening, it enables detailed analysis of amorphous glass networks that cannot be studied using diffraction techniques. This makes it indispensable for modern bioactive glass research.
4. Compatibility with Advanced NMR Methods
MAS can be combined with advanced techniques such as cross-polarization and two-dimensional NMR. These methods improve sensitivity and allow correlations between different atomic species, helping researchers study hydrated surfaces, phosphate groups, and borate units in greater detail.
5. Monitoring Structural Changes Over Time
MAS-NMR allows researchers to track how the glass structure changes during dissolution, ion exchange, and hydroxyapatite formation. Shifts in Qⁿ distributions or modifier environments can be directly linked to biological performance, offering real-time insight into how bioactive glass behaves in physiological conditions.
Conclusion
NMR spectroscopy, particularly solid-state NMR combined with Magic Angle Spinning, plays a central role in bioactive glass research. It provides detailed, element-specific insight into local structure, network connectivity, Qⁿ species distribution, and modifier ion environments in amorphous glass systems.
By revealing how atomic-scale structure controls ion release, dissolution behaviour, and biological interaction, NMR creates a direct link between glass composition and performance. This makes it an essential tool for designing and optimizing bioactive glass materials for biomedical applications such as bone regeneration and tissue repair.
Contact us through Synthera Biomedical social platforms to stay informed about pioneering bioactive glass research and clinical applications. Follow us on Instagram for product launches and research updates. Join the conversation on Facebook to access valuable resources and community news.
Refereces:
1) Ganga shy meena (2014), Basic Concepts, Principles and Applications of NMR Spectroscopy, International Journal of Innovative Research in Science, Vol. 3, Issue 11.
2) Ekaterina Leonova,Isabel Izquierdo-Barba(2008),Multinuclear Solid-State NMR Studies of Ordered Mesoporous Bioactive Glasses,ACS publication.
3) Maas, W. E.; Laukien, F. H.; Cory, D. G. Gradient, high resolution, magic angle sample spinning NMR J. Am. Chem. Soc. 1996, 118 (51) 13085– 13086.
4) N.A. -eesa , N. Karpukhina (2019),Bioactive glass composite for orthodontic adhesives — Formation and characterisation of apatites using MAS-NMR and SEM,Dental material Volume 35, Issue 4.
5) Claudia Turdean-Ionescua, Baltzar Stevensson (2015),Composition-dependent in vitro apatite formation at mesoporous bioactive glass-surfaces quantified by solid-state NMR and powder XRD,Royal society of chemistry, RSC Adv., 2015, 5, 86061-86071.