Fourier Transform Infrared Spectroscopy (FTIR) remains one of the most indispensable analytical techniques in the characterization of bioactive glass systems. For manufacturers, product developers, and clinicians working with regenerative biomaterials, FTIR offers a direct, molecular-level insight into both the bulk silicate network and the surface transformations that drive bioactivity. Because bioactive glass is structurally defined by a disrupted SiO₂-based network modified with Na₂O, CaO, P₂O₅, and other oxide components, even subtle variations in connectivity or bonding configuration can significantly alter ion release, dissolution rates, and hydroxycarbonate apatite (HCA) formation.
FTIR is uniquely capable of tracking these variations in real time — detecting the vibrational fingerprints of Si–O–Si linkages, non-bridging oxygens, phosphate groups, hydroxyls, and emerging carbonate species. These features allow researchers to correlate compositional design with clinical performance, making FTIR essential for both R&D and regulatory documentation.
What Is Bioactive Glass and Why Structure Matters
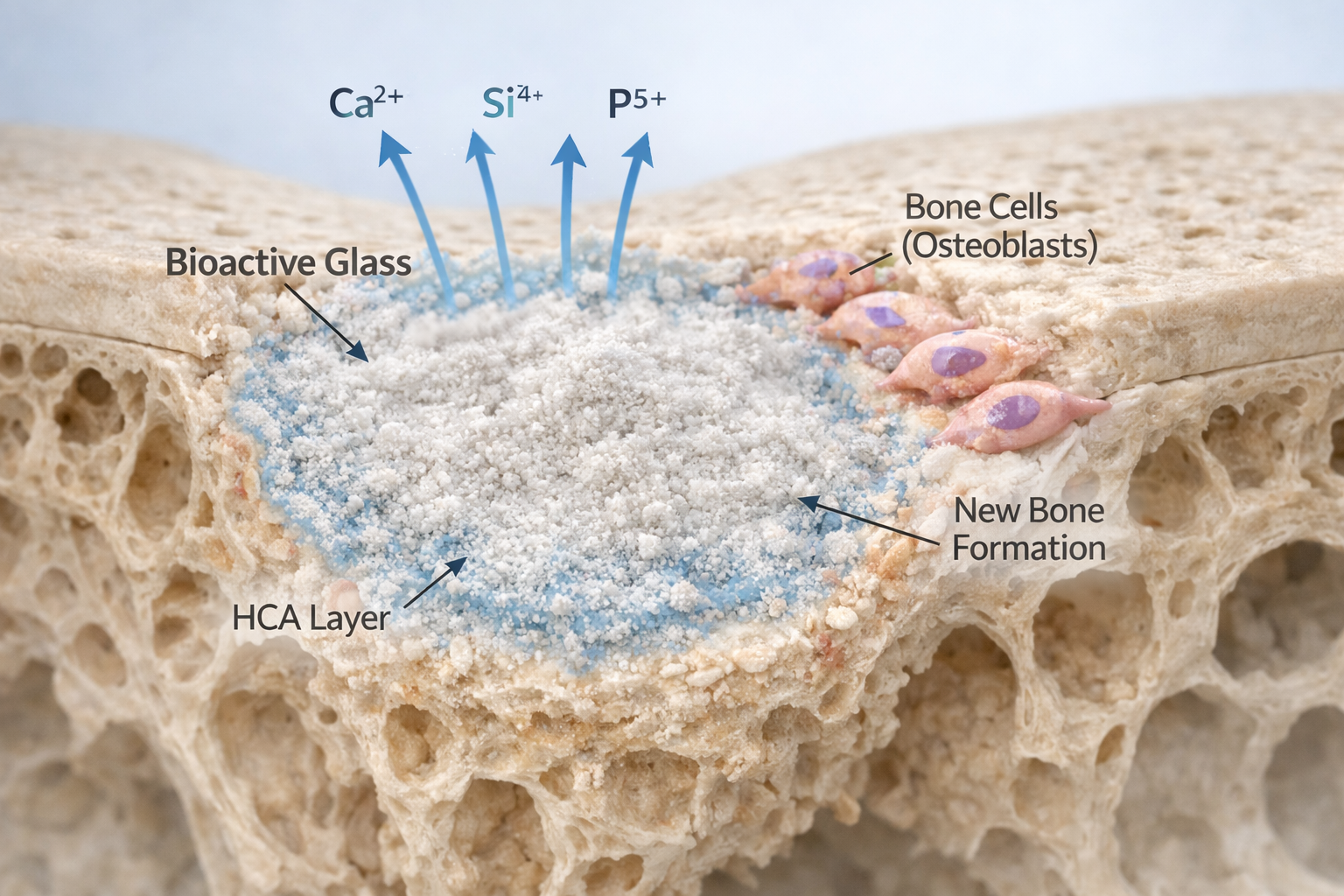
Bioactive glass belongs to a class of surface-reactive, osteoconductive, and osteostimulatory biomaterials originally developed by Larry Hench. Its functional performance — from early ion release to the formation of an HCA layer — is dictated by the silicate network structure.
Bioactive glass is fundamentally distinct from inert glasses because:
- It contains a disrupted silicate network, enabling rapid ion exchange.
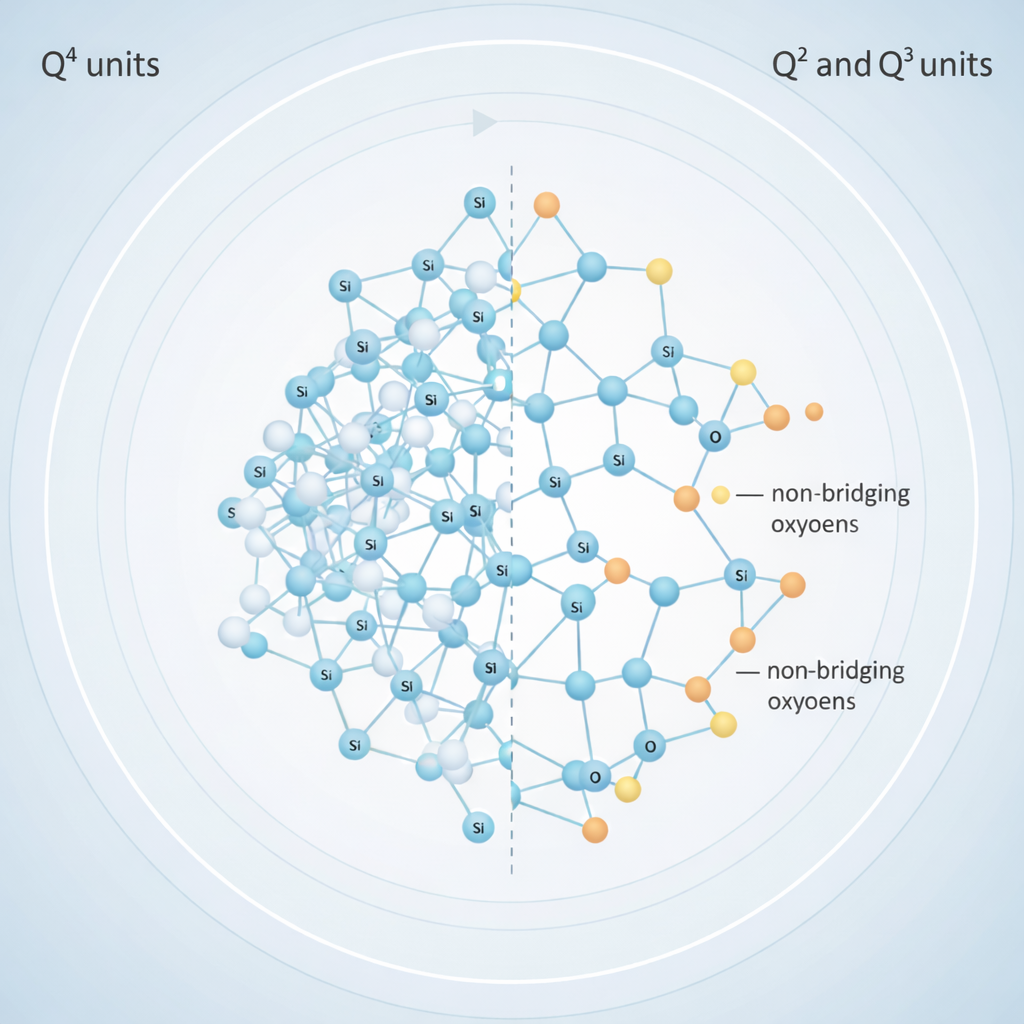
- It incorporates network modifiers (Na⁺, Ca²⁺) that generate non-bridging oxygens (NBOs).
- It includes phosphate groups, which influence early mineralization behaviour.
- It reacts with physiological fluids to form a bone-bonding interface.
The presence and distribution of bridging oxygens (BOs) versus non-bridging oxygens (NBOs) determine how easily the structure depolymerizes in contact with tissue fluids. More NBOs typically correlate with faster dissolution, quicker silica-gel formation, and enhanced bioactivity. FTIR remains the most practical method to evaluate these structural features with precision.
Why FTIR Is Essential in Bioactive Glass Research
FTIR provides spectral information in the 4000–400 cm⁻¹ mid-infrared region, where characteristic vibrational modes of silicates, phosphates, carbonates, and hydroxyls are observed. This region contains the primary fingerprints for evaluating network connectivity and surface reactivity.
Key Spectral Regions of Interest
- 1000–1100 cm⁻¹ — Si–O–Si asymmetric stretching, the main indicator of network polymerization.
- 850–950 cm⁻¹ — Si–O⁻ vibration of non-bridging oxygens, rising with Na₂O/CaO content.
- 550–650 cm⁻¹ — P–O bending modes, representing phosphate groups and early apatite.
- 1400–1500 cm⁻¹ + ~875 cm⁻¹ — CO₃²⁻ carbonate peaks, confirming HCA formation, consistent with bone mineral studies (1).
- 3400 and 1640 cm⁻¹ — OH⁻ absorption, indicating hydration and silica-gel formation.
These features allow researchers to understand the structural baseline of the glass and track transformations during dissolution — directly influencing its performance in dental, orthopedic, and regenerative medical devices.
FTIR and Bioactivity: Tracking the Surface Reaction Sequence
The bioactivity of silicate-based glasses follows a well-characterized five-stage mechanism. FTIR is one of the only techniques that can clearly capture each stage.
1. Ion Exchange and Silanol Formation
Immediately upon immersion in SBF or physiological fluids, modifier cations (Na⁺, Ca²⁺) diffuse out of the glass in exchange for H⁺/H₃O⁺.
FTIR Evidence: Broadening in the ~960 cm⁻¹ band due to increased Si–OH groups (2).
2. Formation of a Hydrated Silica-Rich Layer
As silanol groups undergo condensation, an amorphous, hydrated silica network forms at the surface.
FTIR Evidence: Strong OH⁻ bands at 3400 and 1640 cm⁻¹ (3).
3. Calcium–Phosphate Nucleation
Ca²⁺ released from the glass interacts with phosphate groups, forming amorphous calcium phosphate (ACP).
FTIR Evidence: Broadened 560–600 cm⁻¹ P–O bands (4).
H3: 4. Apatite Maturation
The ACP phase transitions toward crystalline apatite.
FTIR Evidence: Sharper doublets at 564 and 604 cm⁻¹, indicating ordered PO₄³⁻ environments.
5. Hydroxycarbonate Apatite (HCA) Formation
Incorporation of carbonate into the apatite lattice signifies complete maturation of the mineral layer.
FTIR Evidence: Strong ~875 cm⁻¹ out-of-plane CO₃²⁻ bending + 1400–1500 cm⁻¹ stretching (1).
This mineralizing layer is structurally similar to bone mineral, as demonstrated in carbonate-related FTIR analyses of bone mineral (1). For manufacturers, confirming HCA formation validates the bioactivity of their formulation.
ATR-FTIR: A Surface-Sensitive Tool for Tracking Reaction Kinetics
Attenuated Total Reflectance FTIR (ATR-FTIR) is widely preferred for studying surface-specific transformations because it probes only a few micrometres into the material — ideal for monitoring silica-gel formation, Ca–P nucleation, and early HCA.
ATR-FTIR has become a standard tool in bioceramics and biopharmaceutical surfaces due to its sensitivity and minimal sample preparation requirements (3).
Advantages for Bioactive Glass Analysis
- Detects real-time surface reactions.
- Works with hydrated layers, unlike traditional transmission FTIR.
- Requires minimal mass and no KBr mixing.
- Offers high reproducibility for comparative studies.
Key ATR Indicators in Bioactive Glass
- 1000–1100 cm⁻¹: Si–O–Si stretching — decreases in intensity as silicate network dissolves.
- 850–950 cm⁻¹: Si–O⁻ groups — intensity shifts reflect changing NBO content.
- 550–650 cm⁻¹: P–O bands — ideal for observing the onset of calcium phosphate.
- 875 + 1400–1500 cm⁻¹: Carbonate — confirms HCA.
- 3400 + 1640 cm⁻¹: Hydration signatures.
Recent work in endodontic materials demonstrates how ATR-FTIR can classify structural modifications in innovative bioactive glass sealers, supporting their clinical relevance (4).
Transmission FTIR (KBr Pellet): Evaluating Bulk Structure
The KBr pellet transmission method remains one of the best approaches to understanding the intrinsic (bulk) structure of bioactive glasses — before any dissolution or reaction occurs.
It produces sharp, well-resolved absorption bands suitable for compositional comparison and quality control during manufacturing.
Advantages:
- Excellent for characterizing network connectivity.
- Allows comparison of multiple glass formulations.
- Provides high clarity for Si–O–Si and Si–O⁻ assignments.
- Widely reported in bioactive glass literature (2).
Typical Bands Observed
- 1000–1100 cm⁻¹ — Si–O–Si asymmetric stretching
- 850–950 cm⁻¹ — NBO-related Si–O⁻ stretching
- 430–500 cm⁻¹ — Si–O–Si bending
- 550–650 cm⁻¹ — P–O modes
- 1400–1500 + 875 cm⁻¹ — Carbonate peaks (can appear due to atmospheric CO₂)
Transmission FTIR thus complements ATR-FTIR by distinguishing structural features inherent to the glass itself
FTIR as a Tool for Material Development and Quality Control
For manufacturers and developers designing biomedical composites, endodontic sealers, 3D-printed scaffolds, or PLA/bioactive glass filaments, FTIR helps in:
- Monitoring network depolymerization and oxide incorporation.
- Verifying phosphate distribution and reactivity.
- Predicting dissolution behaviour based on NBO content.
- Confirming HCA formation in vitro.
- Supporting regulatory submissions with reproducible spectral evidence.
Recent studies on PLA/BG composite filaments highlight how FTIR validates the presence and integration of glass particles within polymer matrices and tracks interactions during processing (5).
Why FTIR Remains Indispensable
FTIR remains unmatched because it can:
- Identify BO vs. NBO distribution
- Track modifier-induced depolymerization
- Detect phosphate coordination changes
- Monitor hydration and silica-gel formation
- Confirm carbonate incorporation during HCA formation
- Support batch-to-batch quality control
- Provide rapid feedback on composition design
For every new generation of bioactive glass — whether intended for bone regeneration, dental repair, or tissue engineering — FTIR serves as a direct, molecular-level window into how structure determines function
Contact us through Synthera Biomedical social platforms to stay informed about pioneering bioactive glass research and clinical applications. Follow us on Instagram for product launches and research updates. Join the conversation on Facebook to access valuable resources and community news.
References
- Rey C, Collins B, Goehl T, Glimcher MJ (1989) The carbonate environment in bone mineral. A resolution-enhanced Fourier transform infrared spectroscopy study. Calcif Tissue Int 45:157–164
- J. Serra, P. González, S. Liste (2003) FTIR and XPS studies of bioactive silica based glasses. Journal of Non-Crystalline Solids, Volume 332, Issues 1–3.
- Tiernan, Bernadette Byrne (2020) ATR-FTIR spectroscopy and spectroscopic imaging for the analysis of biopharmaceuticals, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Volume 241.
- Guha P, Solete P, Antony D (2025) Microstructural and elemental characterization of novel bioactive glass bioceramic sealer using Fourier transform infrared and X-ray diffraction analysis. J Conserv Dent Endod.
Aditya T N, Ravinder Reddy P (2024) Development and Characterization of PLA/Bioactive Glass Composite Filament for Biomedical Applications.