Bioactive glass are fascinating materials. Unlike conventional inert glasses used in windows or bottles, these glasses are designed to actively interact with the human body. They can bond with bone, stimulate tissue regeneration, and release ions that trigger healing responses. Because of this unique behaviour, bioactive glasses are widely researched for applications in orthopaedics, dentistry, wound healing, and even skincare.
But to truly understand why a bioactive glass behaves the way it does, scientists need to look far deeper than what is visible to the naked eye. The real story lies at the atomic level—how atoms are arranged, how they bond with each other, and how easily they can move or be released. This is where Nuclear Magnetic Resonance (NMR) spectroscopy becomes an indispensable tool.
This blog explains how NMR helps scientists understand bioactive glasses using clear, accessible, and academically grounded language. While the discussion is rooted in peer‑reviewed research, it is written to remain understandable to readers beyond the laboratory. By the end of this article, readers will gain a solid understanding of how NMR reveals the internal structure of bioactive glasses and why this structural knowledge is essential for designing next‑generation materials for orthopaedic, dental, and emerging skincare applications.
Why Studying Bioactive Glass Structure Is Challenging
Most materials we are familiar with—such as metals or crystals—have atoms arranged in highly ordered, repeating patterns. Techniques like X-ray diffraction work very well for such materials because they rely on this long-range order.
Bioactive glasses, however, are amorphous. This means their atoms do not follow a neat, repeating arrangement. Instead, the structure is more like a tangled three-dimensional network. While this disordered structure is exactly what makes bioactive glasses reactive and biologically useful, it also makes them difficult to study using traditional methods.
NMR spectroscopy overcomes this limitation. Rather than looking for long-range order, NMR focuses on the local environment around specific atoms. It can tell us how an atom is bonded, what kind of neighbours it has, and how it moves within the glass network. For amorphous materials like bioactive glasses, this makes NMR uniquely powerful.
What Is NMR, in Simple Terms?
At its core, NMR works by placing a material in a strong magnetic field and observing how certain atomic nuclei respond when exposed to radiofrequency pulses. Some nuclei—such as silicon, phosphorus, sodium, and calcium—behave like tiny magnets. Their response depends on their local chemical environment.
Each type of nucleus gives a different kind of information:
- Silicon (²⁹Si) tells us about the backbone of the glass network
- Phosphorus (³¹P) reveals how phosphate groups are arranged and how bone-like minerals form
- Sodium (²³Na) provides insight into ion mobility and glass reactivity
- Calcium (⁴³Ca) helps explain how calcium participates in healing and mineral formation
By combining information from all these nuclei, scientists can build a complete picture of how a bioactive glass is structured and how it will behave inside the body.
²⁹Si NMR: The Backbone of Bioactive Glasses
Silicon is the main building block of most bioactive glasses. It forms tetrahedral units (SiO₄) that link together through oxygen atoms, creating the glass network.
Understanding Network Connectivity
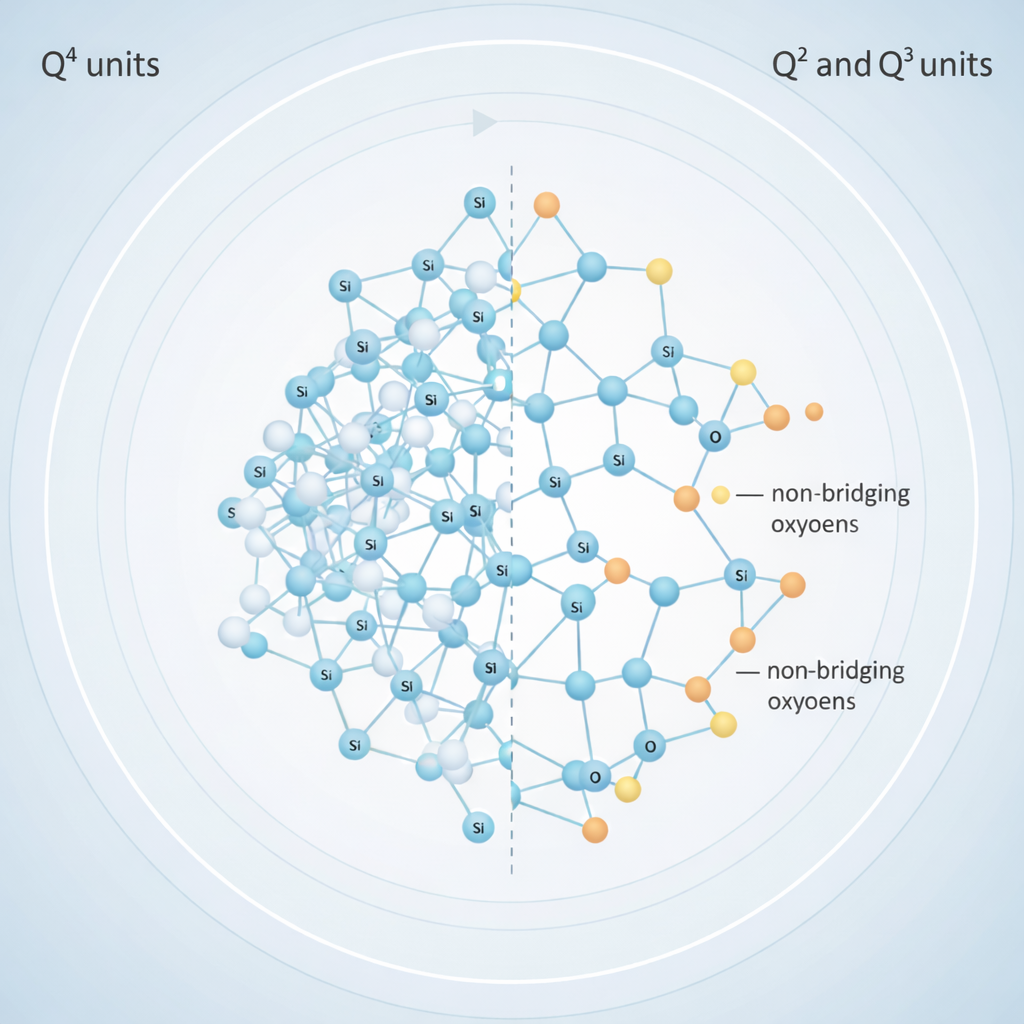
One of the most important concepts revealed by ²⁹Si NMR is network connectivity, often described using the Qⁿ notation. Here, “n” refers to the number of oxygen atoms shared with neighbouring silicon atoms:
- Q⁰: isolated units
- Q¹: end-of-chain units
- Q²: chain or ring structures
- Q³: sheet-like structures
- Q⁴: fully connected three-dimensional networks
Highly connected networks (rich in Q⁴ units) are very stable but dissolve slowly. Bioactive glasses, on the other hand, intentionally contain more Q¹, Q², and Q³ units. This partially broken network allows the glass to dissolve in bodily fluids and release beneficial ions.
Why This Matters for Bioactivity
Using ²⁹Si NMR, researchers can directly measure how connected the glass network is. This helps predict:
- How fast the glass will dissolve
- How quickly ions will be released
- How easily a bone-like layer can form on the surface
²⁹Si NMR is also used to study how processing steps—such as sintering or sterilisation—change the glass structure, and how the network evolves when the glass is immersed in simulated body fluid.
³¹P NMR: Linking Glass Structure to Bone Formation
Phosphorus plays a central role in the bioactivity of glasses, especially in bone-bonding applications. In the body, phosphorus combines with calcium to form hydroxyapatite, the mineral phase of natural bone.
Phosphate Environments in Glass
³¹P NMR allows scientists to distinguish between different phosphate environments:
- Isolated phosphate groups, which are common in many bioactive glasses
- Linked or polymerised phosphates, which behave differently during dissolution
The technique can also reveal whether phosphate groups are evenly distributed throughout the glass or clustered in specific regions.
Watching Bone-Like Minerals Form
One of the most powerful uses of ³¹P NMR is monitoring what happens when bioactive glass is placed in simulated body fluid. As the glass reacts, sharp and well-defined phosphorus signals often appear. These signals are a clear sign that crystalline calcium phosphate—closely related to natural bone mineral—is forming on the glass surface.
This makes ³¹P NMR a direct bridge between atomic-scale structure and real biological performance.
²³Na NMR: Understanding Ion Release and Reactivity
Sodium is not part of the glass backbone, but it plays a crucial supporting role. It acts as a network modifier, breaking some of the silicon–oxygen bonds and creating sites that make the glass more reactive.
What Sodium Tells Us
²³Na NMR provides insight into:
- How sodium ions are coordinated within the glass
- How evenly they are distributed
- How much disorder exists around modifier sites
Because sodium ions are relatively mobile, variable-temperature NMR experiments can even track how easily they move within the glass network.
Why Sodium Mobility Is Important
The mobility of sodium ions is directly linked to:
- Initial glass dissolution
- Ion exchange with body fluids
- Surface reactions that trigger bioactivity
By understanding sodium behaviour, scientists can fine-tune glasses for faster or slower reactions, depending on the intended medical application.
⁴³Ca NMR: A Window into Calcium’s Role in Healing
Calcium is one of the most biologically important elements in bioactive glasses. It supports bone growth, influences cell behaviour, and plays a key role in forming hydroxyapatite.
A Technically Challenging Nucleus
Studying calcium using NMR is difficult. The ⁴³Ca isotope is rare and produces weak signals. As a result, advanced instruments and specialised techniques are required.
What We Learn from ⁴³Ca NMR
Despite these challenges, ⁴³Ca NMR provides unique information about:
- How many oxygen atoms surround calcium
- Whether calcium mainly modifies the network or balances charges
- How calcium environments change during glass dissolution and mineral formation
This information is invaluable for understanding how calcium contributes to both structural stability and biological performance.
Why Multinuclear NMR Is So Powerful
No single NMR experiment can explain everything about a bioactive glass. The real strength of NMR lies in combining information from multiple nuclei:
- ²⁹Si explains the glass backbone
- ³¹P links structure to bone-like mineral formation
- ²³Na reveals ion mobility and reactivity
- ⁴³Ca clarifies calcium’s functional role
Together, these insights allow researchers to design bioactive glasses with specific dissolution rates, controlled ion release, and enhanced biological responses.

From Atomic Structure to Real‑World Applications
Understanding the atomic‑scale structure of bioactive glasses is not an academic exercise alone. Insights gained from NMR spectroscopy directly inform how these materials are designed, processed, and applied in real clinical and commercial settings.
In orthopaedics, controlling silicate network connectivity and calcium coordination allows researchers to tailor dissolution rates and ion release profiles that support bone regeneration and implant integration. In dentistry, phosphate speciation and early apatite formation—clearly revealed by ³¹P NMR—are central to developing glasses that promote enamel remineralisation and dentin bonding. Emerging research in skincare and soft‑tissue repair increasingly draws on similar principles, where controlled ion release and surface reactivity influence cellular responses, angiogenesis, and tissue rejuvenation.
Crucially, NMR enables these applications without relying on trial‑and‑error formulation. By linking atomic environments to functional outcomes, researchers and industry stakeholders can rationally design bioactive glasses that are safer, more predictable, and better suited to their intended biological context.
Conclusion: NMR as the Cornerstone of Bioactive Glass Research
NMR spectroscopy has transformed our understanding of bioactive glasses by providing direct access to their atomic‑scale structure. Through multinuclear approaches involving ²⁹Si, ³¹P, ²³Na, and ⁴³Ca, NMR reveals how glass networks are built, how modifiers behave, and how biologically relevant phases form during interaction with physiological environments.
For biomaterials researchers, this structural insight enables rational composition design and processing control. For medical, dental, and skincare stakeholders, it translates into materials with predictable performance and enhanced biological functionality. Ultimately, NMR does not merely characterise bioactive glasses—it underpins their intelligent design, firmly establishing it as one of the most critical tools in contemporary bioactive glass research.
Contact us through Synthera Biomedical social platforms to stay informed about pioneering bioactive glass research and clinical applications. Follow us on Instagram for product launches and research updates. Join the conversation on Facebook to access valuable resources and community news.
Reference:
1) Stevensson, B., Mathew, R., & Edén, M. (2014). Assessing the phosphate distribution in bioactive phosphosilicate glasses by ³¹P solid-state NMR and molecular dynamics simulations. Journal of Physical Chemistry B, 118(30), 8863–8876. https://doi.org/10.1021/jp504601c
2) Lockyer, M. W. G., Holland, D., & Dupree, R. (1995). NMR investigation of the structure of some bioactive and related glasses. Journal of Non-Crystalline Solids, 188(3), 207–219. https://doi.org/10.1016/0022-3093(95)00134-4
3) Eckert, H. (2018). Structural characterization of bioactive glasses by solid-state NMR. Journal of Sol-Gel Science and Technology, 88(2), 263–295. https://doi.org/10.1007/s10971-018-4748-1
4) Angeli, F., Villain, O., Schuller, S., Ispas, S., & Charpentier, T. (2011). Insight into sodium silicate glass structural organization by multinuclear NMR combined with first-principles calculations. Geochimica et Cosmochimica Acta, 75(9), 2453–2469. https://doi.org/10.1016/j.gca.2011.02.022
5) Bryce, D. L. (2010). Calcium binding environments probed by ⁴³Ca NMR spectroscopy. Dalton Transactions, 39(36), 8593–8602. https://doi.org/10.1039/C0DT00416B
6) Lin, S., Ionescu, C., Pike, K. J., Smith, M. E., & Jones, J. R. (2009). ⁴³Ca and ²⁹Si MAS NMR evidence for calcium environments during bioactive glass dissolution and apatite formation. Chemistry of Materials, 19(6), 1276–1285. https://doi.org/10.1021/cm061622h