Invitro cytotoxicity testing
In vitro cytotoxicity testing is a fundamental step in the biological evaluation of bioactive glasses, especially in the context of biomedical applications such as bone regeneration, tissue engineering, and implantable devices. These tests aim to assess whether the material or its degradation products have toxic effects on cultured cells, which serves as an early predictor of its compatibility with living tissues.
The core concept involves exposing cultured mammalian cells to either the bioactive glass material directly or to its extracts, which are prepared by incubating the material in a culture medium. The resulting cellular responses, such as viability, metabolic activity, and membrane integrity, are quantified using biochemical assays and microscopic observation. These responses help determine whether the material exhibits toxic effects at specific concentrations or under particular conditions. (1)
Two standard controls are used for comparison:
- Positive Control (e.g., phenol): A known cytotoxic substance used to validate that the assay can detect a toxic effect.
- Negative Control (e.g., alumina): A biologically inert material used to represent a non-toxic baseline.
Protocol for In Vitro cytotoxicity testing:
Sample Preparation
- Form Factor: Bioactive glass is typically fabricated as discs, granules, powders, or particles depending on the intended application.
- Sterilization: Samples are sterilized to prevent microbial contamination. Methods include ultraviolet (UV) radiation, autoclaving, or ethanol soaking, depending on the material’s heat and chemical sensitivity.
- Characterization: Prior to testing, physical and chemical properties (e.g., surface area, porosity, ion release profile) may be characterized, as they influence cytotoxic responses.
Extract (Indirect Contact Method)
- Conditioned Medium Formation: Bioactive glass samples are immersed in cell culture medium (e.g., DMEM or RPMI) at a specified surface area-to-volume ratio (e.g., 3 cm²/mL or 200 mg/mL) for periods ranging from 24 hours to 7 days.
- Simulating Physiological Conditions: The incubation is carried out under standard cell culture conditions (37°C, 5% CO₂) to simulate body-like environments.
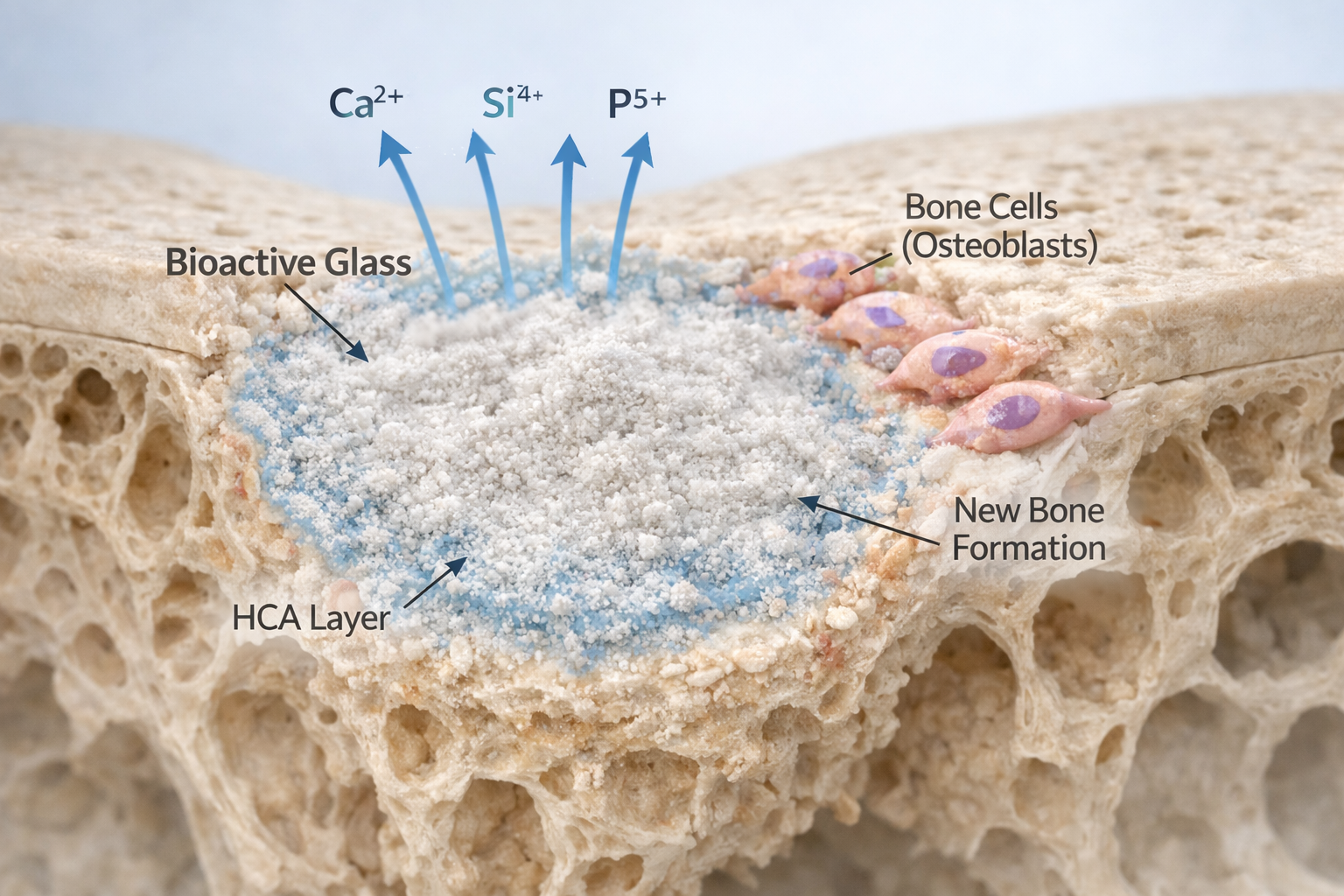
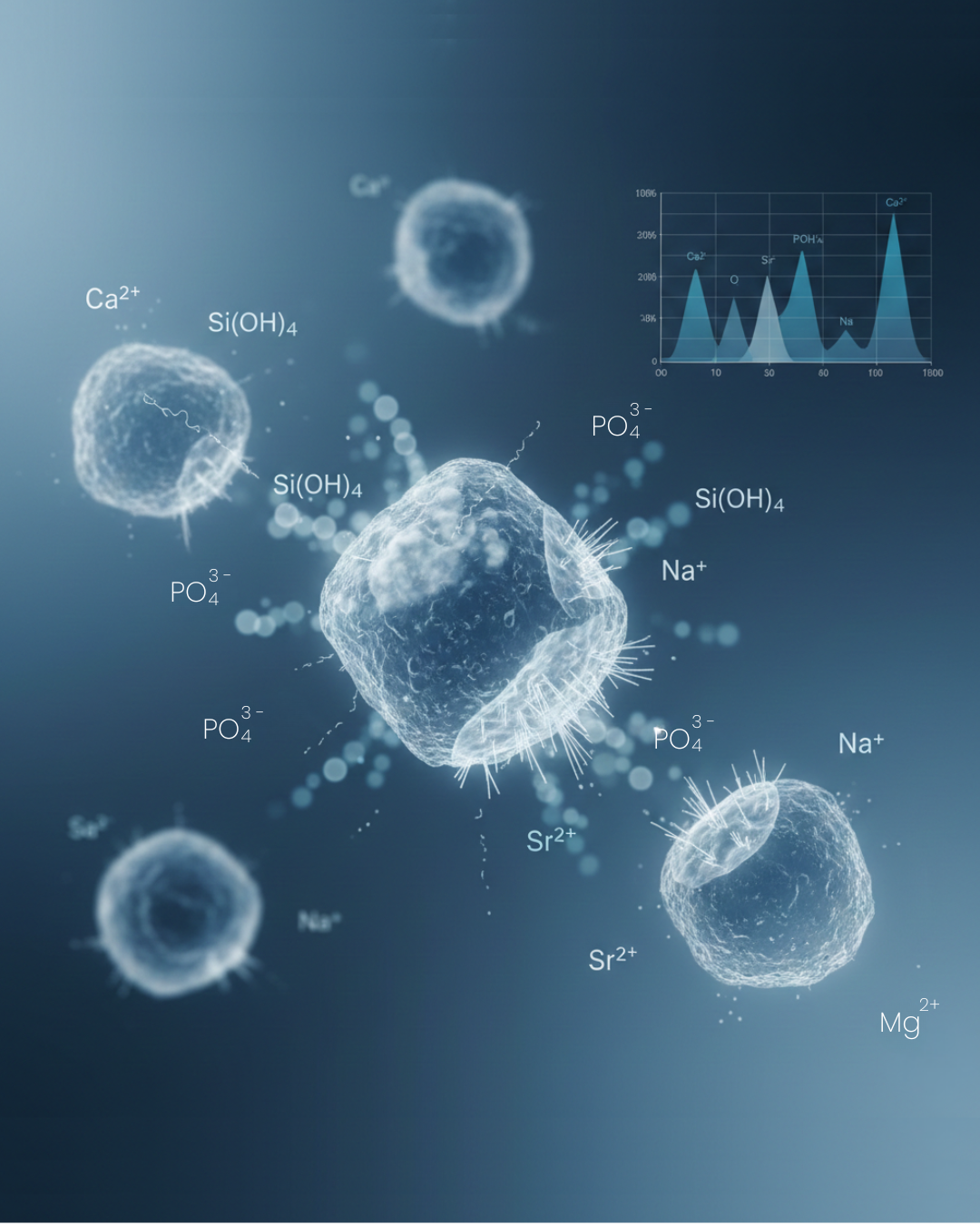
- Serial Dilutions: The resulting extract, now containing ions such as Ca²⁺, Si⁴⁺, Na⁺, and P⁵⁺, is filtered and diluted to prepare a range of concentrations for dose-dependent studies.
Cell Culture and Exposure
The selection of appropriate cell lines is a critical step in in vitro cytotoxicity testing of bioactive glass, as it ensures the biological relevance of the results based on the material’s intended clinical application. For general biocompatibility assessments, fibroblast cell lines such as L929 are commonly used due to their robustness and standardization across toxicity studies. For bone-related applications, osteoblasts or osteoblast-like cells such as MG-63 and SaOS-2 are preferred, as they more accurately reflect the cellular environment where bioactive glass would function. In more advanced regenerative medicine studies, human mesenchymal stem cells (hMSCs) are employed because of their multipotent nature and sensitivity to material-induced effects. Once the appropriate cells are selected, they are seeded into 96-well plates and allowed time to adhere and proliferate under standard culture conditions. After this incubation period, the cells are exposed to the bioactive glass either through the indirect method using pre-prepared material extracts or via direct contact with glass particles or discs. To ensure the reliability and reproducibility of the experiment, both positive controls (using known cytotoxic agents) and negative controls (untreated cells) are included. These controls provide reference points for interpreting the degree of cytotoxicity observed and confirm the assay’s sensitivity and accuracy. (2)
Cytotoxicity Assessment Techniques
Multiple assays are used to evaluate different aspects of cell health:
- MTT Assay
The MTT assay is a colorimetric method used to assess cell viability based on metabolic activity. The principle of the assay relies on the ability of living cells to reduce the yellow tetrazolium salt, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide), into insoluble purple formazan crystals. This reduction is facilitated by mitochondrial dehydrogenase enzymes, which are active only in metabolically functional (viable) cells. After incubation with MTT, the resulting formazan crystals are solubilized—typically using a solvent such as dimethyl sulfoxide (DMSO)—to allow quantification. The absorbance of the solubilized formazan is then measured using a spectrophotometer at a wavelength of 570 nm. The intensity of the colour directly correlates with the number of viable cells: higher absorbance indicates greater cell viability and metabolic activity. Conversely, a decrease in absorbance reflects reduced metabolic activity and, therefore, lower cell viability, which may be indicative of cytotoxic effects of the tested substance or material. This makes the MTT assay a widely used and reliable method for evaluating cytotoxicity in biomaterial testing, including for materials like bioactive glass. (3)
- Neutral Red Uptake (NRU) Assay
The principle of this assay lies in the selective uptake of the dye by living cells through active transport and its accumulation in the acidic environment of the lysosomes. This process depends on intact cell membranes and functional lysosomal activity. When cells are damaged or stressed, particularly when the plasma or lysosomal membranes are compromised, they lose the ability to sequester the dye, resulting in reduced uptake. After incubation with the neutral red solution, excess dye is removed, and the retained dye is extracted from the cells using a solvent. The amount of dye released is then quantified spectrophotometrically by measuring absorbance, typically at around 540–550 nm. The absorbance directly reflects the number of viable cells with intact lysosomal membranes. A decrease in absorbance compared to untreated controls indicates a loss of lysosomal integrity and a reduction in cell viability, thus suggesting potential cytotoxic effects of the tested material or compound. This assay is commonly used alongside other viability tests to assess the biocompatibility of biomaterials such as bioactive glass. (4)
C. Microscopic Evaluation
- Cell Morphology: Cells are visually inspected under a microscope to assess shape, adherence, and confluence.
- Signs of Toxicity: Rounded cells, detachment, vacuolization, or membrane blebbing are indicative of cellular stress or death. (4,5)
Data Analysis and Interpretation
In cytotoxicity testing, quantitative analysis involves normalizing the assay results to a negative control (usually untreated cells), with the data expressed as a percentage of cell viability. This allows for easy comparison between treated and untreated groups. To further evaluate the potency of a test material, the IC₅₀ value—defined as the concentration at which 50% of cell viability is inhibited—is determined from dose-response curves. This metric provides insight into the material’s cytotoxic threshold. To ensure the reliability of the results, statistical analyses such as ANOVA or t-tests are conducted to determine whether the differences between groups are statistically significant, thereby validating the observed cytotoxic effects.
References:
- Rismanchian M, Khodaeian N, Bahramian L, Fathi M, Sadeghi-Aliabadi H. In-vitro Comparison of Cytotoxicity of Two Bioactive Glasses in Micropowder and Nanopowder forms. Iran J Pharm Res. 2013 Summer;12(3):437-43.
- Elena Mancuso, Oana A. Bretcanu, Martyn Marshall, Mark A. Birch, Andrew W. Novel bioglasses for bone tissue repair and regeneration: Effect of glass design on sintering ability, ion release and biocompatibility,Materials & Design,Volume 129,2017,Pages 239-248.
- Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced osteoclast-like cell functions on nanophase ceramics. Biomaterials. 2001;22:1327–1333. doi: 10.1016/s0142-9612(00)00285-4.
- Li W, Zhou J, Xu Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed Rep. 2015 Sep;3(5):617-620.
- Riss T, Niles A, Moravec R, et al. Cytotoxicity Assays: In Vitro Methods to Measure Dead Cells. 2019 May 1.
Contact us through Synthera Biomedical social platforms to stay informed about pioneering bioactive glass research and clinical applications. Follow us on Instagram for product launches and research updates. Join the conversation on Facebook to access valuable resources and community news.