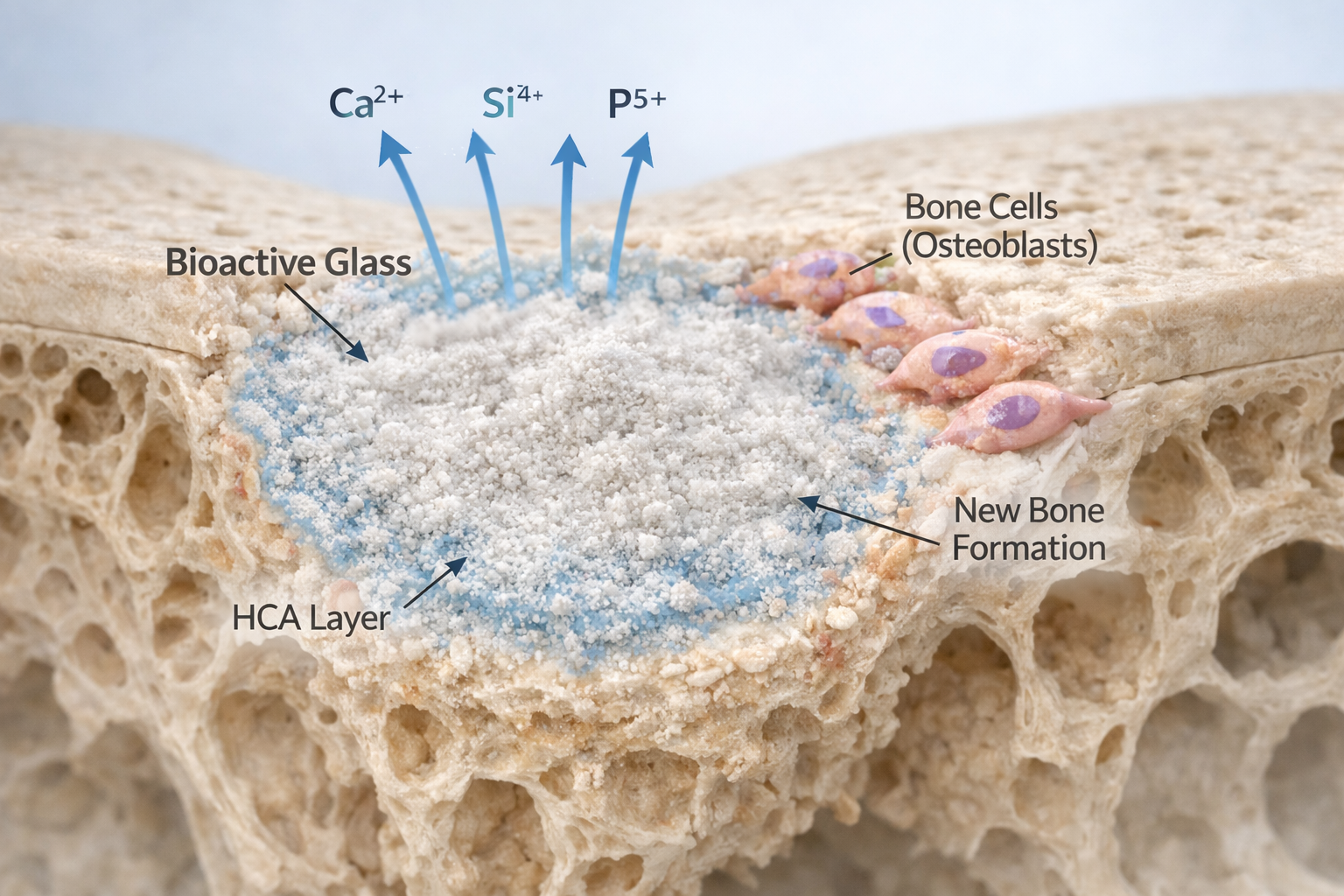
Bioactive glass is one of the most ground-breaking innovations in the field of biomedical materials. Since its invention in the late 1960s, it has proven to be a versatile and highly effective material for a wide range of medical applications from repairing bone defects and promoting tissue regeneration to serving as a carrier for targeted drug delivery. Its ability to form a direct chemical bond with living tissues makes it uniquely bioactive, setting it apart from traditional inert materials used in implants and grafts.
The method of preparation plays a critical role in determining the glass’s internal structure, surface area, porosity, and overall reactivity with biological systems. A change in preparation technique can significantly alter how the glass behaves inside the body, how fast it dissolves, and how effectively it bonds with bone or releases therapeutic agent. (1)
Melt Quenching Method: The Original Approach
The melt quenching method is the traditional and most widely used technique for producing bioactive glass. It was the process behind the first-ever formulation of bioactive glass—45S5 Bioglass®, developed in the late 1960s. This method involves mixing raw materials such as silicon dioxide (SiO₂), sodium carbonate (Na₂CO₃), calcium carbonate (CaCO₃), and phosphorus pentoxide (P₂O₅) in precise proportions. The mixture is then melted at high temperatures, typically between 1300°C and 1500°C, in a platinum or high-purity crucible until a homogeneous molten glass is formed.
Once melted, the glass is rapidly cooled or “quenched” by pouring it onto a cold steel plate or into water. This quick cooling prevents the formation of crystals, resulting in an amorphous (non-crystalline) glass structure. The solidified glass is then often crushed and ground into fine powder, especially if it is intended for use in coatings, composites, or other applications requiring specific particle sizes.
One of the major advantages of the melt quenching method is its simplicity and scalability, making it suitable for mass production. It also produces glass with relatively good mechanical strength, which is important for structural and load-bearing applications. However, the method has some limitations. Because of the high processing temperatures involved, it is energy-intensive and may not be suitable for incorporating temperature-sensitive components like certain drugs or proteins. Additionally, the resulting glass typically has a low surface area and limited porosity, which can reduce its bioactivity compared to glasses made by other methods like sol–gel processing.
Despite these drawbacks, melt quenching remains a cornerstone technique in the production of bioactive glasses, especially where mechanical strength and manufacturing scalability are prioritized. (2)
Sol–Gel Method: Chemistry Meets Nanotech
Sol–Gel Method: Chemistry Meets Nanotech
The sol–gel method is a versatile and widely used approach in the fabrication of nanostructured and porous bioactive glasses, offering exceptional control over both composition and structure. This method begins with the hydrolysis of alkoxide precursors, such as tetraethyl orthosilicate (TEOS), in either an acidic or basic aqueous solution. The hydrolysed solution forms a colloidal suspension, or “sol,” which gradually undergoes gelation to create a three-dimensional gel network. Once gelation is complete, the material is carefully dried and subjected to heat treatment at relatively low temperatures (around 600°C) to remove residual solvents and organic materials, resulting in a highly porous, amorphous bioactive glass.
One of the most significant advantages of the sol–gel method is that it produces glass with a high surface area and well-controlled porosity, especially mesopores (pores between 2 and 50 nanometres in diameter). These features are ideal for enhancing bioactivity and for applications such as drug delivery and tissue engineering, where porosity plays a key role in cell infiltration and nutrient exchange. Additionally, the sol–gel process operates at much lower temperatures than melt quenching, making it suitable for incorporating sensitive bioactive molecules.
However, the sol–gel process is more complex and time-consuming compared to traditional methods. The mechanical strength of sol–gel-derived glass is also typically lower unless additional densification steps are applied. Despite these challenges, the sol–gel method is a powerful tool for tailoring bioactive glasses for advanced biomedical applications. (3)

Spray Pyrolysis: For Ultrafine Particles
Spray pyrolysis is an advanced technique used to produce ultrafine, spherical bioactive glass particles, particularly suited for applications like implant coatings and drug delivery systems. The process starts by preparing a precursor solution, typically containing metal salts or alkoxides. This solution is then atomized into fine droplets using a nozzle and carried through a high-temperature furnace (typically 300–1000°C). As the droplets pass through the heated zone, they undergo rapid thermal decomposition, forming fine glassy particles that can be collected and used directly.
This method offers several benefits, including the production of uniform and monodisperse particles with controlled morphology. It is also scalable and continuous, making it attractive for industrial manufacturing. However, spray pyrolysis can be equipment-intensive and offers less compositional flexibility compared to methods like sol–gel. Additionally, the rapid heating may limit the incorporation of temperature-sensitive additives. (4)
Foam Replication and 3D Printing: For Porous Scaffolds and Bone Mimics
Modern fabrication techniques like foam replication and 3D printing have opened up new possibilities for producing macroporous bioactive glass scaffolds that closely resemble the structure of natural bone. These methods are particularly promising in the field of bone tissue engineering, where interconnected porosity and architectural control are essential for supporting cell growth, nutrient diffusion, and vascularization.
In foam replication, a porous polymer foam (such as polyurethane) is coated with a bioactive glass slurry. Once dried, the structure is subjected to high temperatures that burn out the foam template, leaving behind a highly porous glass scaffold. In contrast, 3D printing allows for digital control over the scaffold design. Using bioactive glass powders or inks, structures are printed layer-by-layer and then sintered to form solid, porous constructs.
These techniques offer the advantage of custom-designed porosity and complex geometries, making them ideal for personalized implants and bone defect repair. However, the processes can be technically complex and may require further optimization to improve mechanical strength, particularly in load-bearing applications. (5)
Conclusion:
The method of preparation plays a pivotal role in defining the physical, chemical, and biological characteristics of bioactive glass, directly impacting its effectiveness in various medical applications. Each technique offers distinct advantages and limitations, making them suitable for specific purposes.
the choice of preparation method must align with the intended biomedical application, balancing factors such as porosity, mechanical strength, processing complexity, and compatibility with bioactive agents. This strategic selection ensures the optimal performance of bioactive glass in its role as a transformative biomaterial in modern medicine.
References:
Fiume E, Ciavattini S, Verné E, Baino F. Foam Replica Method in the Manufacturing of Bioactive Glass Scaffolds: Out-of-Date Technology or Still Underexploited Potential? Materials (Basel). 2021 May 24;14(11):2795. doi: 10.3390/ma14112795. PMID: 34073945; PMCID: PMC8197364.
Kargozar S., Baino F., Hamzehlou S., Hill R.G., Mozafari M. Bioactive Glasses: Sprouting Angiogenesis in Tissue Engineering. Trends Biotechnol. 2018;36:430–444. doi: 10.1016/j.tibtech.2017.12.003.
Regi MV. Ceramics for medical applications, Dalton Trans 2001;
2:97–108.
Hench LL, West JK. The sol-gel process. Chem Rev 1990;90:33–72
Tseng, C.-F.; Fei, Y.-C.; Chou, Y.-J. Investigation of in vitro bioactivity and antibacterial activity of manganese-doped spray pyrolyzed bioactive glasses. J. Non-Cryst. Solids 2020, 549, 120336.